Introduction: Acute myeloid leukemia (AML) poses significant economic burden on the healthcare system, particularly during induction therapy and at disease relapse. The burden associated with ongoing post-remission therapy is less clear. In patients diagnosed with AML enrolled in Medicare who received an induction therapy and achieved disease remission, we assessed the healthcare resource utilization (HCRU) and costs associated with each disease period (induction, early/late post-remission, and post-relapse).
Methods: A retrospective analysis was conducted using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, comprising Medicare claims (parts A, B, and D from 2007 to 2016) and the US National Cancer Institute's SEER database (cancer diagnoses from 2007 to 2015). Identified patients had a diagnosis of AML in the SEER registry, were ≥ 65 years at the AML diagnosis date, initiated chemotherapy post-AML diagnosis (i.e. induction), and had an International Classification of Diseases, Ninth/Tenth Revision (ICD-9/10) diagnosis code for AML remission following the start of therapy. Patients were excluded if they had another blood malignancy, had received a prior hematopoietic stem cell transplant, or were enrolled in a clinical trial.
Induction was defined as any therapy received from the date of first post-diagnosis chemotherapy initiation (index date) to the end of the cycle during which a patient had an ICD-9/10 code for AML remission. The 6 months prior to the index date was defined as the baseline period. Post-remission therapy was divided into an early post-remission period, which included any therapy initiated within the first 60 days (≤ 60 d) after end of induction, and a late post-remission period, which included therapy initiated more than 60 days (> 60 d) after end of induction. If specific treatment information was available, late post-remission therapy was defined by a treatment switch occurring > 60 d after end of induction. Post-remission therapy ended at the earliest of relapse or end of follow-up (i.e., death, end of eligibility, or end of available data [December 31, 2016]). The post-relapse period was from the date of first AML relapse ICD-9/10 code after remission to the end of follow-up.
Baseline patient characteristics, as well as HCRU and costs (adjusted to 2019 US dollars) during the baseline, induction, post-remission, and post-relapse periods, were summarized descriptively. HCRU and costs associated with induction and post-remission therapy periods were assessed during days that were part of a treatment cycle. The average per patient per month (PPPM) HCRU and costs were reported. Duration of response (DoR) from the first remission to the earliest of relapse or death was estimated using Kaplan-Meier analysis.
Results: A total of 530 patients were identified. The median age at AML diagnosis was 73 years, 53.6% of patients were male, and 80.6% were white. The median time from index date to the end of follow-up was 13.5 months. Most patients received therapy with hypomethylating agents during the AML treatment.
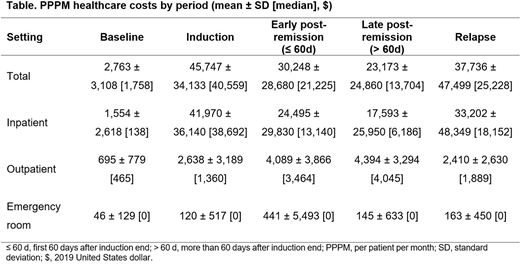
A total of 31.9% of patients who achieved remission did not receive post-remission therapy during follow-up; for these patients, mean (median) time from end of induction to relapse or end of follow-up was 125 (23) days. A total of 63.2% of patients received chemotherapy in the early post-remission period, and 43.0% of all patients went on to receive chemotherapy in the late post-remission period. The median DoR was 5.8 months; a total of 48.9% of patients had relapsed and 80.2% had died by the end of follow-up. The mean PPPM healthcare costs were highest for induction, followed by post-relapse, early post-remission, and late post-remission periods (Table). Costs associated with the inpatient (IP) setting were the greatest contributor to PPPM costs across all periods. IP visits were most common during induction with 92.1% of patients having ≥ 1 IP visit, relative to 53.8% during baseline, 65.7% during early post-remission, 71.5% during late post-remission, and 91.1% post-relapse.
Conclusions: The economic burden of relapse is approximately 1.2 and 1.6 times higher than the mean PPPM healthcare costs during early and late post-remission periods, respectively. There exists a large unmet need for therapies that will extend the duration of the post-remission period and reduce the overall economic burden of AML.
Tabah:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Huggar:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Karyopharm Therapeutics: Current equity holder in publicly-traded company; FibroGen: Current equity holder in publicly-traded company. Huey:Bristol Myers Squibb: Current Employment. Copher:Bristol Myers Squibb: Current Employment. Zhou:BMS: Other: Employee of Analysis Group Inc., which received consulting fees. Zichlin:BMS: Other: Employee of Analysis Group Inc., which received consulting fees. Koenigsberg:BMS: Other: Employee of Analysis Group Inc., which received consulting fees. Brunner:Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; AstraZeneca: Research Funding; Forty-Seven Inc: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.